 Laboratory
9
Laboratory
9
Arthropod
Sensory Receptors
Recording action potentials from the cercal nerve of Periplaneta americanus
Objectives
- To detect and record action potentials on nerve fibers with extravehicular placed electrodes.
- To observe the effects of different kinds of stimulation on the neural activity in the central nervous system.
- To produce and test a hypothesis on any aspect of cercal function in the American cockroach
Introduction
Activity of the nervous system underlies all behavioral phenomena. First, a receptor must respond to some environmental change. In doing so, it generates action potentials on nerve fibers leading to the central nervous system (CNS). In the CNS, signals are received by inter neurons which integrate these signals and initiate a response in accordance with the information being received. Finally, action potentials encoding the response are sent to effectors--muscles or glands--to produce the appropriate behaviors. The nervous system is thus analogous to a telephone communications network. The central switching station of the telephone system, which directs incoming calls to the designated receivers, is like the CNS, while the telephone wires carrying the signals are like the nerve fibers to and from the CNS.
Nerve action potentials are electrical voltage changes that move smoothly along the nerve fiber membrane, rapidly, yet not nearly so fast as the essentially instantaneous pulses of electricity on a telephone wire. In this experiment, you will attempt to detect action potentials on sensory neurons going into the CNS, and on inter neurons within the CNS, Because they are active in the dark, cockroaches (Periplaneta americana) have especially sensitive touch receptors and chemoreceptors. The former are particularly abundant in the two small prongs extending out on either side of the posterior end of the abdomen, the abdominal cerci (singular = cercus). These cerci are so sensitive to air pressure and bending that a weak puff of air is enough to cause the roach to jump reflexively, and run away. You will attempt to detect and characterize the nerve action potentials in response to puffs of air against the cerci. Once you can record this response you can use this experimental model to investigate cercal function in the roach. (auditory - sound frequency and intensity; tactile - physical contact; chemosensory - which molecules; pressure waves - intensity; threshold and saturation for any stimulus; nature of the receptor - tonic or aphasic; or anything else that appears interesting.
Preparation of Ventral Nerve Cord:
- Fortunately I have devised a technique that eliminates all of the prep work required to obtain recording from the roach ventral nerve cord. If you want to record directly from the cercal nerve, however, this preparation will be required.
- Decapitate the roach by quickly snipping off its head. Then clip off the wings, and legs, to prevent it from running away or producing movement artifacts in the recordings. (At least they are easier to catch after decapitation).
- If you are recording from the ventral nerve only, skip forward to
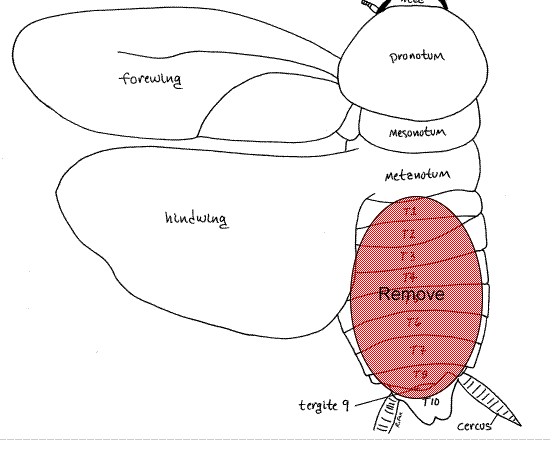 For access to the cercal nerve
For access to the cercal nerve
- Pin the roach ventral side down to the dissecting pad. It is best to put the pad directly on the bench top, rather than to leave it in the plastic tray. This will steady the preparation after the electrodes are in place on the nerve cord (see below).
- Dissect off the abdominal terga (the dorsal abdominal plates) and remove the digestive organs to expose the ventral nerve cord and cercal nerves (Figures 1).
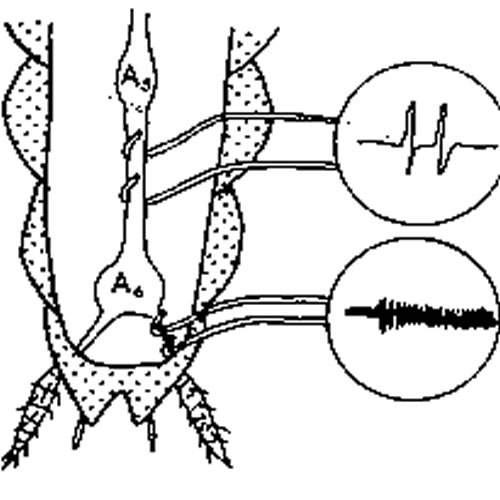
- Be careful not to stretch or pin the ventral nerve cord. Immediately fill the body cavity with saline-mineral oil mixture that has been shaken for five minutes. Position the two (positive and negative) wire electrodes about 1 mm apart gently under the nerve cord (Figure 2). When the electrodes are in place,use a tiny lump of modeling clay to steady the electrodes.

-
The third electrode (the ground) can be inserted anywhere in the abdomen.
 Recording from the ventral nerve chord requires no dissection
Recording from the ventral nerve chord requires no dissection
- Pin the roach to the dissecting pad ventral side up
- Insert the electrodes as indicated in fig 3.
- The ventral nerve chord is very close to the surface of the abdomen so you should attempt to keep the electrodes close to the surface.
- Insert the electrode nearly vertically 1 to 2 mm from the center line. Do not penetrate more than a mm
- Once the cuticle is breached, flatten out the electrode to near horizontal and push it to about 1 to 2 mm on the other side of the nerve
- Now angle the electrode up so that it will exit on the other side of the ventral nerve chord
Experimental Procedure:
- Set up your MP35 and SS58L stimulator So that the stimulator is on channel 1 and the roach on channel 2
- Connect the recording electrodes to the MP35 with BSLCBL
- Ignore the shields
- Connect lead closest to the but to VIN-
- Copy the template roachButTemp to your desk top and open BPSLpro by double clicking the template.
- Make certain that the stimulator output is 0 volts
- Click start and check that all is well
 Click start again and blow gently on the cerci with a pipette
Click start again and blow gently on the cerci with a pipette
- You should see a train of action potential elicited by the stimuli
- Auto scale the axis and check that your gain is set appropriately
- Do the following experiment: Determine the frequency of action potential spikes, and duration of a burst of spikes, when a brief puff of air is delivered to the cerci.
- Note the effect on frequency of action potentials and duration of bursts when you blow continuously on the cerci.
Produce and test hypotheses about roach cerci.
- If you are interest in examining threshold sub maximal and supra maximal stimuli, you can use the stimulator to deliver precise clicks by attaching the headphones to the stimulator.
- Find the Sony mini plug/RCA jack cable an attach
the BNC adapter to the RCA end and the Sony adapter to the Sony end. Attach the BNC adapter to the stimulator output and the headphones to sony adapter. The head phones can now be placed about a cm from the cerci and
frequency and magnitude of the clicks produced can be controlled with the stimulator.
- This set up can be used to investigate threshold, fatigue and latency of the receptors and the phasic or tonic
nature of the receptors.
-
- If you are interested in examining the frequency response of the cerci you can use perfect pitch (lab 1) to out put various frequencies at various volumes
- If you are interested in examining pressure waves (like an approaching predictor) you can swat at the preparation with a note book. This is difficult to quantify but you can categorize the stimului - high med and low intensity. Remember the importance of missing the roach when you swat.
- You can also explore direct tactile responses or odors or light or heat or anything you can think of
Report.
You are Performing experiments on this preparation so a full lab report would be appropriate. Dont forget the function of a methods section as methods are abreviated in this write up.
Literature.
Bell, William J. 1981. The laboratory cockroach, experiments in cockroach anatomy, physiology and behavior. Chapman and Hall, London and New York.
Barnes, Robert D. 1974. Invertebrate zoology, third edition. W. B. Saunders Company, Philadelphia, London, Toronto.
Walter I. Hatch
wihatch@smcm.edu
November 19, 2012
 Laboratory
9
Laboratory
9 Laboratory
9
Laboratory
9 For access to the cercal nerve
For access to the cercal nerve


 Recording from the ventral nerve chord requires no dissection
Recording from the ventral nerve chord requires no dissection
 Click start again and blow gently on the cerci with a pipette
Click start again and blow gently on the cerci with a pipette


