 Laboratory
8
Laboratory
8 Laboratory
8
Laboratory
8Molluscan hearts are said to be "myogenic" since the pacemaker system for heartbeat resides in the muscle making up the heart. Cardioregulator nerves, however, modify the frequency and amplitude of beat through the release of neurohumoral agents. In most mollusks, acetylcholine appears to be the mediator of the inhibitor fibers to the heart and there is growing evidence that 5-hydroxytryptamine (5-HT, serotinin, enteramine) mediates the action of the cardioexcitor nerves. In some mollusks, at least, the inhibitor and exciter fibers to the heart run in the same nerve or nerves. It is therefore difficult to stimulate, selectively, one set of fibers and then the other. However, indirect methods may be used to show that two sets of fibers are present. One of these consists of employing chemical agents that specifically block one or the other neurohumoral agent. The purpose of this experiment is first to determine the effect of stimulating the cardioregulator nerves (or ganglion from which they arise) and then to use appropriate pharmacological agents to mimic, or attenuate the response to nerve stimulation. The results of this experiment should allow you to speculate on the nature of the cardioregulator transmitter substances.
Note: you will need your dissection kit
It will be difficult to interpret the results of your experiments unless you have a basic understanding of the equipment that you will be using to gather the data.
This initial procedure will allow you an opportunity to become familiar with the basic function of the equipment will confirm that the equipment is operational and eliminate equipment malfunction as a potential source of difficulty during your experiments.
The study of cardiac activity used to involve mechanically coupling the beating heart to a lever capable of writing on a paper covered drum. The moving lever would scratch a trace in a thin layer of soot deposited on the paper. This procedure is messy, difficult to adjust and dangerous as the soot is produced from burning benzene, a powerful carcinogen. You will be using an electronic transducer to convert the weak mechanical displacements of the beating heart into electrical signals. These signals can then be amplified and displayed on either an oscilloscope,strip-chart recorder or the more contemporary A/D converter and computer graphics interface. Its safe, easy and only 4 K$ a unit. Treat the transducers with care or its back to burning benzene.
Reposition the lever so that left side extends 100 mm out from the fulcrum. You will move this end up and down 100 mm. The easiest way to accomplish this is to move the entire transducer down so that the axil is about 12 mm from the desk top so that you can move it both up and down 100 mm. Calibration can now accomplished by opening the scaleing window (from inside the setup window). Enter the Scale values +10 and -10 and change the units to mm so that they corrispond to the distance that the lever would move at the point of attachment of the thread (1cm from the fulcrum). Now simply move the left end of the lever up 100mm (resulting in the right end at 10 mm mark moving down 1mm). While the lever is held in that position click the cal 1 button. Move the right end down 100mm and click the calb 2 button. That's it its calibrated. It is a good idea to check the results by starting an acquisition and moving the right end up and down 1000mm. The graph should indicate a 10.0 mm movement at the 10mm mark on the left end of the lever.
 At this point you should
At this point you should
move the lever so that the weight of the lever produces a slight tension on the thread.
Large, bivalve mollusks are suitable for this experiment, including such genera as Mercenaria, Mya, Spisula, Schizothaerus and certain of the fresh water mussels. Members of the genus Mytilus behave differently from other bivalves that have been studied. The direction and comments to follow apply specifically to Mercineria mercenaria.
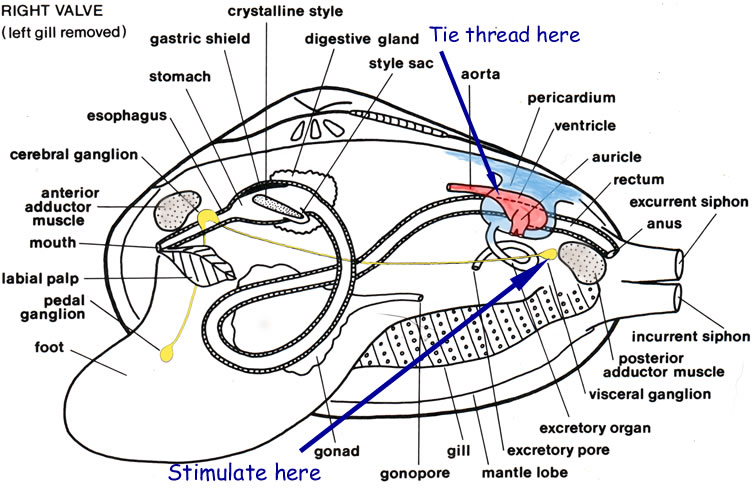
Mount the preparation, intact valve downward, on a mound of Plasticine in a plastic dissecting pan (remember that the dissecting pans equipped with blue rubber pads in this laboratory are for live animals only). Keep the umbo away from you and slant the valve slightly so that fluids drain off its margin. Attach a thread to the heart by tying a light thread loosely around the atrio-ventricular junction. Attach the thread to the right end of the light heart lever exactly 10 mm from the fulcrum.
Adjust the position of the heart lever within the lever clamp so that the weight of the left side of the lever provides slight tension in the thread.
Remember that the distance from the fulcrum of the lever and the point of attachment of the thread will determine the sensitivity of the setup and if the attachment moves from the 10mm value that you used for calibration you will need to re calibrate.
Set the time window in AcKnowledge for approximately 2 minutes (long enough to get a representative sample but not so long that you can not interpret individual contractions. adjust the various parameters so that you can produce a clear recording of cardiac activity. Save a copy of your best record using the file conventions from the data acquisition lab. Be certain to enter the experimental parameters into the notebook section of Acknowledge for this and all other experiments.
Note You might fid it easier to save all of your .acq on your desktop and then move only the best of them to archimedes for inclusion into the report.
Set up a sea-water-filled, non-polarizable electrode. The cathode should be placed on the ganglia, and the anode electrode, a silver wire, should contact the mantle edge. Alternatively set up a twin pole platinum stimulating electrode and position the leads so that there is one on either side of the ganglia. Set the stimulus duration to about 1 msec and the stimulus frequency at 100/sec. Stimulus for 5 seconds and observe the effect on the heartbeat. In the event of no effect on the amplitude or frequency of heartbeat, increase voltage by steps until there is an effect, or make necessary checks and adjustments.
If possible, find a stimulus that will give complete stoppage of heartbeat for a period of about 15 sec. More intense stimuli are not advisable. Observe a few runs of your experiments and note the pattern of recovery of the beat after stopping the stimulation. Is there a temporary increase in frequency? If so, how might you account for it? Save your best record for electrical stimulation as before.
While recording the heart beat as in 5 above, apply a few drops of 10-6 M acetylcholine (Ach) to the heart.
Rinse with ringers until the beat is similar to the undisturbed animal and save a new control data set. Now apply a few drops of 10-6 M 5 Hydroxytripamine (5HT) to the heart.
Carlson, A. J., 1905. Comparative physiology of the invertebrate heart. II. The function of the cardiac nerves in mollusks. Am. J. Physiol., 13:396-426.
Carlson, A. J., 1906. Comparative physiology of the invertebrate heart. VII. The relation between the intensity of the stimulus and the magnitude of the contraction. Am. J. Physiol., 16:85-99.
Carlson, A. J., 1905. Comparative physiology of the invertebrate heart. Biol. Bull., 8:123-168.
Luduena, F. P. and T. G. Brown, Jr., 1952. Mytolon and related compounds as antagonist of acetylcholine on the heart of Venus mercenaria. J. Pharmacol. Exptl. Therap., 105:232-239.
Prosser, C. L., 1940. Acetylcholine and nervous inhibition in the heart of Venus mercenaria. Biol. Bull., 78:92-102.
Welsh, J. H., 1956. Neurohormones of invertebrates. I. Cardioregulators of Cyprina and Buccinum. J. Mar. Biol. Assoc. U. K., 35:193-201.
Welsh, J. H., 1957. Serotonin as a possible neurohumoral agent: evidence obtained in lower animals. Ann. N. Y. Acad. Sci., 66:618-630.
Welsh, J. H. and R. Taub, 1948. The action of choline and related compounds on the heart of Venus mercenaria. Biol. Bull., 95:346-353.
Welsh, J. H. and R. Taub, 1953. The action of acetylcholine antagonists on the heart of Venus mercenaria. Brit. J. Pharmacol., 8:327-333.
Walter I. Hatch
wihatch@smcm.edu
November 12, 2012