 Laboratory
02
Laboratory
02 Laboratory
02
Laboratory
02Prelaboratory Preparation Required
The osmotic concentration of a solution depends on the total number of solute particles, irrespective of kind. Many animals conform osmotically to their environment but can regulate specific ion concentrations in their internal environment. In no organism are the extracellular concentrations and intracellular concentrations of ions identical. One of the most universal qualities of living things is the selection at the cell surface of specific ions and the exclusion of others. At the cellular and organismic levels, ion regulation is a general and primitive capacity. It is your goal in this exercise to examine the ion concentration of three euryhalinic estuarine organisms in relation to the ion concentration of their surrounding media. From this data you can draw conclusions as to the level of ion regulation these organisms are capable of. Is it at the cellular level or do these organisms regulate their internal fluid environment? (Data on ion composition of a variety of organisms can be found on page 80 of Prosser's Comparative Animal Physiology as well as in your text book)
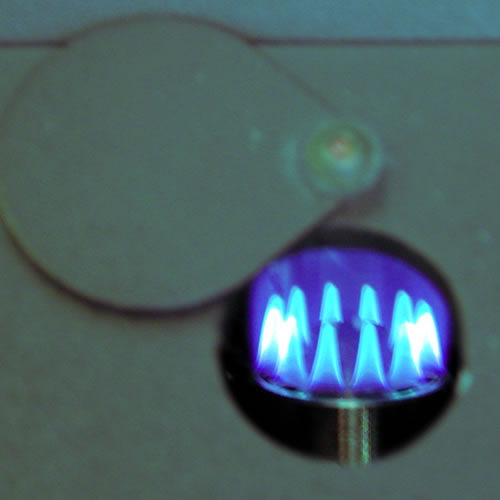
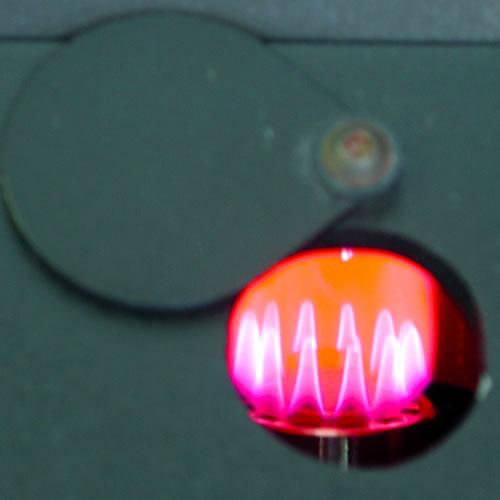 One
highly accurate method of determining the concentration of specific ions
makes use of the fact that ionized metals will emit and absorb very specific
wave lengths of light. This makes possible the use of flame and atomic
absorption photometers which excite the metals within a flame and then
measure the emittance or absorbance or specific wave lengths of light.
As the intensity of the light is proportional to the concentration, and
the wave length specific for a specific ion, both quantitative and qualitative
data can be obtained. Notice the color emmited as lithium is aspirated
into a flame in the figures above. You will use a Corning digital clinical
flame photometer to measure the concentrations of sodium and potassium,
and calcium, in this exercise. This instrument is designed for quantification
of a few clinically important ions. In order to reduced its cost and make
it more accessible to broadly trained clinical technicians (as opposed
to highly specialized AA technicians) it lacks the sophistication and
versatility of Atomic absorption or emission instrumentation. The instrument
is ,thus, subject to some limitations that you should be aware of of (see
the flame photometer appendix). In spite of fact that it lacks the
sophistication of an AA spectrophotometer it is still a delicate and expensive
unit. Read the instructions and treat it with care.
One
highly accurate method of determining the concentration of specific ions
makes use of the fact that ionized metals will emit and absorb very specific
wave lengths of light. This makes possible the use of flame and atomic
absorption photometers which excite the metals within a flame and then
measure the emittance or absorbance or specific wave lengths of light.
As the intensity of the light is proportional to the concentration, and
the wave length specific for a specific ion, both quantitative and qualitative
data can be obtained. Notice the color emmited as lithium is aspirated
into a flame in the figures above. You will use a Corning digital clinical
flame photometer to measure the concentrations of sodium and potassium,
and calcium, in this exercise. This instrument is designed for quantification
of a few clinically important ions. In order to reduced its cost and make
it more accessible to broadly trained clinical technicians (as opposed
to highly specialized AA technicians) it lacks the sophistication and
versatility of Atomic absorption or emission instrumentation. The instrument
is ,thus, subject to some limitations that you should be aware of of (see
the flame photometer appendix). In spite of fact that it lacks the
sophistication of an AA spectrophotometer it is still a delicate and expensive
unit. Read the instructions and treat it with care.
Observe the labels on the crab condominiums in the SH wet lab and place in in each experimental salinity at least 48 hours prior to the experiment. Each replicate will require:
If each team gets a sample from each organism we will have six replicates. Or you may work as a super team with each lab team sampling only one type of organism. Either way remember your report won't be convincing with out statistical analysis so think about how many replicates you will need.
The pugnacious nature and cannibalistic tendencies of the blue crab requires some special precautions if they are to be maintained in close quarters. Any method of preventing the use of their chelipeds will work, but the removal of the cheliped is your best insurance. The decapod crustacea have the ability to automize limbs if their retention would threaten the safety of the organism. You need only threaten the crab by holding the cheliped in a Bunsen burner flame and the distressed crab will contract specialized muscles that will drop the claw and seal off the circulatory system. Failure to disable or remove the chelipeds will threaten the success of everyone's experiment. It is not necessary or advisable to disarm fiddlers.
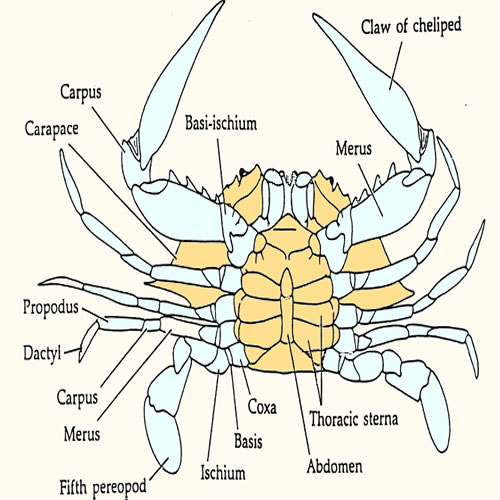 Collect
at least (250 ul) (1/4 microfuge tube) of
blood by piercing the membrane between fifth
periopod and body with a 3 ml syringe and 16-18 gauge needle. Transfer
the blood immediately to a micro centrifuge tube and stir it vigorously
to promote clotting prior to centrifuging. Alternatively, swiftly cut through
the coxa of the fifth periopod and collect the blood from stump directly
into a 1500ml microfuge tube.
If you utilize this technique, it is critical to avoid contamination
of your sample with water draining from the branchial chamber by wrapping
the animal in a paper towel. Stir the blood vigorously to promote clotting
prior to centrifuging. Smaller animals will require modification of
this technique. Fiddlers are best sampled by cutting basi-ischium of
the large cheliped of males and then pumping the dactyl to express the
blood. Shrimp will not provide much blood. cutting through the abdomen
just posterior to the heart and collecting blood directly into hematocrit
tubes should work.
Collect
at least (250 ul) (1/4 microfuge tube) of
blood by piercing the membrane between fifth
periopod and body with a 3 ml syringe and 16-18 gauge needle. Transfer
the blood immediately to a micro centrifuge tube and stir it vigorously
to promote clotting prior to centrifuging. Alternatively, swiftly cut through
the coxa of the fifth periopod and collect the blood from stump directly
into a 1500ml microfuge tube.
If you utilize this technique, it is critical to avoid contamination
of your sample with water draining from the branchial chamber by wrapping
the animal in a paper towel. Stir the blood vigorously to promote clotting
prior to centrifuging. Smaller animals will require modification of
this technique. Fiddlers are best sampled by cutting basi-ischium of
the large cheliped of males and then pumping the dactyl to express the
blood. Shrimp will not provide much blood. cutting through the abdomen
just posterior to the heart and collecting blood directly into hematocrit
tubes should work.
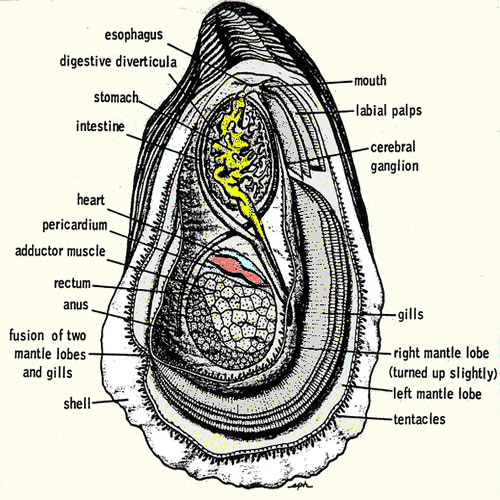 Collect
blood by carefully open the oyster and reflect the mantle away from
the dorsal body wall. The heart
should be visible, beating within the pericardial cavity. Drain the
mantel cavity and blot the pericardium dry with tissue and carefully
pierce the membrane with fine tipped forceps, reflect the membrane and
collect as much blood as possible (at least 250 ul) with a narrow bore
disposable pipette. Transfer the blood to a 1500 ml microfuge tubes.
If you damage the pericardial membrane while opening the oyster, you
can still collect an uncontaminated sample by shaking the all of the
fluid from the oyster and then waiting for thee pericardial cavity to
refill.
Collect
blood by carefully open the oyster and reflect the mantle away from
the dorsal body wall. The heart
should be visible, beating within the pericardial cavity. Drain the
mantel cavity and blot the pericardium dry with tissue and carefully
pierce the membrane with fine tipped forceps, reflect the membrane and
collect as much blood as possible (at least 250 ul) with a narrow bore
disposable pipette. Transfer the blood to a 1500 ml microfuge tubes.
If you damage the pericardial membrane while opening the oyster, you
can still collect an uncontaminated sample by shaking the all of the
fluid from the oyster and then waiting for thee pericardial cavity to
refill.
 Unfortunately you can not kill the fish first as a beating heart is required
to pump the blood out of the aorta. MS-222(Tricaine methane-sulfonate) is,
however an efective fish anesthetic and you shoud immerse your fish in a
100mg MS222/l of culture water. Immerse the fish for about 10 min prior to
collecting the blood and return them to a leathal solution of 250mg/l when
you have compleated the proceedure. Work as quickly and humanly as possible.
A pooled blood sample from three fish should be enough for both osmotic pressure
and ion concentration measurements.
Unfortunately you can not kill the fish first as a beating heart is required
to pump the blood out of the aorta. MS-222(Tricaine methane-sulfonate) is,
however an efective fish anesthetic and you shoud immerse your fish in a
100mg MS222/l of culture water. Immerse the fish for about 10 min prior to
collecting the blood and return them to a leathal solution of 250mg/l when
you have compleated the proceedure. Work as quickly and humanly as possible.
A pooled blood sample from three fish should be enough for both osmotic pressure
and ion concentration measurements. All vertebrate blood samples must be centrifuged prior to osmometry or freezing as cellular disruption will liberate variable amounts of osmotically and ionically active material, affecting the results of this experiment as well as varying ionic concentrations that will alter the results of next week's experiment. Invertebrate blood will have significantly fewer cells, but if all samples are to be treated alike you should centrifuge them as well as your water samples.
For these relatively large samples you will use an Eppendorff microfuge. Make certain that the samples are in 1500 ml microfuge tubes and that the caps are tightly sealed. To insure that the rotor remains balanced, place the tubes in the microfuge such that each tube has a tube with equal volume directly across from it. The relatively low speed and low sample volume of the microfuge make it unnecessary to weigh each tube but careful eyeball balance is necessary. Replace the rotor lid, close the cover, and spin the samples for five minutes at full speed. The size and shape of these microfuge tubes allow you to decant the supernatant with out disturbing the pellet. Remember that storage of the supernatant over the pellet may lead to contamination of the sample.
Your fish blood samples are in hematocrit tubes and require a different centrifuge. You should cap one end of the tube with a critocap or critoseal and place in the hematocrit centrifuge (capped end to the outside) in a balanced arrangement. Replace the cover and spin for 5 minutes. Remove the critocap, and very carefully break off the portion of the tube containing cells and other sedimented debris. Blow the contents out into a 1500 ml tube for subsequent use.
A technique as sensitive as emission flame photometry can be fully exploited only by meticulous sample preparation. Glassware which is not clinically clean, and dilutions which are not as accurate as they can possibly be made will increase your sample variance and make it more difficult to to interpret your results.All that is usually necessary is a 200:1 aqueous dilution (10ml/2000ml or20ml/4000ml) of all samples, and the preparations of standards of the required concentrations; but the simplicity of the process should not be allowed to affect its precision. It is also necessary to remember that a clinical flame photometer is designed for use on mammalian (ie. human) body fluid and the dilution factors suggested by its manufacturer may be inappropriate for invertebrates, especially poikiloosmotic invertebrates. If your readings fall off scale on the instrument, it will be necessary to select other dilutions and correct the readings appropriately.
The instrument that you are using has a useful range of measurement between 0 and 200 mmol/L for each of the ions under consideration. If your reading exceeds 200 you should re dilute to a more appropriate concentration and repeat the measurement. Remember the instrument is expecting a one to two hundred dilution (or the same dilution factor used in calibration of the instrument) and its output will be displayed for this dilution factor. You must correct your readings for any changes in the dilution factor that you may find necessary.
Using the protocol found in Flame phometer appendix, run all of your unknown samples and record yor data in mM. Keep careful note of your dilutions factors so that you can back calculate to the actual concentration. Note: everything gets diluted 1 to 200 so do not report this in your dilution factor. For example if you make a 1 to 400 dilution the dilution factor is 2. What you are essentially doing is reportintg the difference between the expected 1 to 200 and the delivered 1 to 400 whic is, of course 2. Remember that the dilution factor is the number you multipy your reading by to obtain the actual concentration
You should enter all of your data on the shared resource spreadsheet Ionregulation. This is a shared resource and each time you save it it will be updated for everyone as well as updating itself with the work of the rest of the class. Because it is a shared resource it is possible for you to damage or over wright the data of others. Carefully enter your data into the appropriate cells.
You should submit a combined lab report on the data collected in this experiment and in lab 1. As the objective here is to learn to produce laboratory reports by incorporate the suggestions for improvement found on your first laboratory report into this report. It will not be due until one week after you receiving comments on your first report. Report ionic concentrations in meq/l of each specific ion and show how body fluid levels relates to concentration in seawater. Draw specific conclusions as to the ability of each species to regulate the specific ion concentration of its body fluid. Does this ability seem related to its osmoregulating ability? Compare each organisms ability to regulate ions in body fluid with their ability to regulate ions within individual cells.
Boon, D.B. 1972. Blood osmotic concentrations of blue crabs (Callinecties) sapidus found in fresh water. Chesapeake Science, 13(4):318-335.
Flemester, L. J. 1958. Salt and water anatomy, consistency and regulation in related crabs from marine and terrestrial habitats. Biol Bull., 115:180-200.
Gilles, R. 1975 Mechanisms of ion and osmo regulation. In Marine Ecology, Vol II, Part I. O. Kinne (Ed) pp. 259-335
Lent C. M. 1969. Adaptations of the Ribbed mussel, Modiolus demissus to the intertidal habitat. Am. Zoo., 9:283-292.
Prosser, C. L. 1973. Comparative Animal Physiology Section I Environmental Physiology - Water: Osmotic Balance; Hormonal Regulation. W. B. Saunders, Philadelphia
Welsh, J. H., R. I. Smith and A. E. Kammer. 1968. Laboratory Exercises in Invertebrate Physiology, Burgess, Minneapolis,
Walter I. Hatch
wihatch@smcm.edu
August 12, 2012