 Laboratory
6
Laboratory
6
Compound
Action Potentials
Conduction
velocity, thresholdand drug effects in isolated nerve
No prelaboratory preparation
required but reading these instructions is a good idea
Objectives
- To familiarize students with the methodology of neurophysiological
investigation.
- To record the CAP of the crab brachial nerve and measure the latent period.
- To investigate the effect of subthreshold, threshold, submaximal, maximal, and
supramaximal stimulation of the brachial nerve on the amplitude of the CAP.
- To investigate the effects of temperature on the velocity of nerve impulse conduction.
- To investigate the threshold voltage, strength duration curve, reobase,
chronaxy, and conduction velocity of a nerve.
- To observe and record nerve refractoriness by stimulating the nerve using maximal stimuli of increasing frequency.
- To investigate the effect of a local anaesthetic on nerve function and speculate on its mode of actinon.
- To interpret the data produced in each of the above investigations
and integrate it into a report discussing the functions of axons in
compound nerves.
Introduction
The sciatic nerve of frogs has been traditionally used by neuropsychologists
and generations of students to study the properties of nerve action potentials.
The ventral nerve of a policheate like Glycera or even the radio-ulnar
nerve of Homo sapiens make acceptable substitutes and eliminate the need to further decimate frog populations. The brachial nerve of large decapod crustacea such as Callinecties sapidus, however, yeilds an even beter preperation for the study of isolated nerve. You will be
recording what is termed a compound action potential detected extracellularly.
Because a nerve is composed of many individual nerve fibers, all of which
may be activated by an electrical stimulus, the extracellular recording
will represent the sum of all of the action potentials on all the active
nerve fibers. This summation of the action potentials on numerous individual
axons is, thus, described as a compound action potential .
To measure extremely rapid and weak electrical changes characteristic
of nerve action potentials, used to use electronic equipment to amplify
the weak signals and to present them in a time frame that can be interpreted.
The wave of electrical activity resulting from the passage of action potentials
is picked up by electrodes contacting the nerve and first amplified by
a preamplifier after which they are sent to the oscilloscope where the
signal causes the proportional deflection of an electron beam. The beam
in turn shines as a spot on the inside face of the cathode ray tube, causing
the phosphor coating to glow. The glowing spot traces a line across the
screen to depict the voltage changes of the nerve.
You will use a the more contemporary BioPac analog to digital (A/D) converter
which will allow not only an analysis of the evoked potentials in the
nerve but the storage of the information on a computer disk for subsequent
analysis. This is a particularly handy feature as the total duration of
an action potential is measured in milliseconds and thus requires some
attention to detail in order to measure or even observe in real time. The A/D
converter and computer simulated oscilloscope "freeze" time
and observe the same experiment repeatedly. This equipment will allow
you to determine the threshold voltage, conduction velocities, and strength
duration curves. With care it will permit an examination of the several
"classes" of axons with different conduction velocities as well as provide you with the means for examining the effect of an anesthetic on nerve functin.
Background
Intracellular Action Potential recordings can give precise information about individual cells, but they are difficult to perform, and beyond the capabilities of most undergtraduate labs. Extracellular recording techniques are much easier to perform, but require a clear understanding of the technique inorder t interpret the reult. The Crab brachial nerve is essentially a bundle of hundreds of individual unmyelinated nerve fibers. The nerve contains both efferent and afferent fibers, so impulse propagation normally occurs in both directions. Even though voltage changes will be introduced and recorded from outside of the nerve, its internal nerve fibers will be indirectly involved because it is made up of conductive fluids. The series of diagrams below explain the CAP recording. In these diagrams, single nerve fibers are shown in yellow. At rest, they have a positive external polarity with respect to a negative internal polarity. If an adequate (threshold or greater) stimulus is applied to the nerve fiber, an action potential will be generated at the site of stimulus. This action potential creates a nerve impulse, which consists of a traveling wave of depolarization followed immediately by a wave of repolarization. Once generated, this impulse will propagate along the fiber away from the stimulus site without change in
amplitude or velocity. Viewed from the outside of the nerve fiber, the impulse begins by creating a negative polarity shift at the region of stimulation, shown in blue. The impulse proceeds as a wave of negative polarity along the outside of the fiber. The diagrams show a voltmeter connected externally to the nerve fiber to record the voltage difference across two points. A graph will be plotted of Voltage vs. Time inside the voltmeter beginning when the stimulus first generates an action potential. It is important to note that the voltmeter reads the potential difference between its positive ("+") and negative ("-") terminals (subtracts "-" from "+"). If the nerve fiber is at rest, the voltmeter will read 0 because both of its terminals are at the same voltage potential. If the voltmeter's "-" terminal is more negative than its "+" terminal, the voltmeter will indicate a positive (upward) voltage.
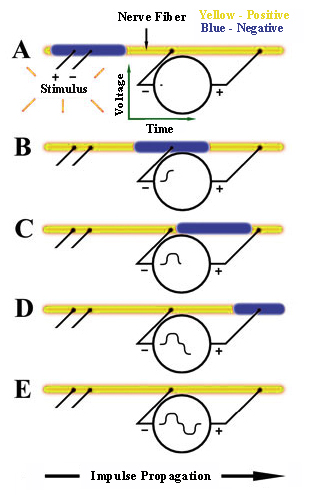 The MP3X will be used to record the voltage difference between two electrodes with respect to time and simultaneously record the voltage across the stimulating electrodes. The diagram to the right shows five "snap-shots" in time of events occurring on a single nerve fiber beginning with A and ending with E. The recording is considered biphasic because the the voltmeter will record both a positive and negative deflection.
The MP3X will be used to record the voltage difference between two electrodes with respect to time and simultaneously record the voltage across the stimulating electrodes. The diagram to the right shows five "snap-shots" in time of events occurring on a single nerve fiber beginning with A and ending with E. The recording is considered biphasic because the the voltmeter will record both a positive and negative deflection.
- When an external stimulus that exceeds a certain threshold level is applied, an action potential will occur,
creating the nerve impulse shown in blue. Since the voltmeter's "-" and "+" terminals at this instant are at the
same voltage potential, it records 0 Volts (A).
- The nerve impulse will propagate along the nerve with a constant amplitude and velocity until it eventually passes through the voltmeter's "-" terminal, causing it to record a positive (upward) voltage reading (B).
- Propagation will continue and a point may be reached where the nerve impulse is between the voltmeter's "-" and "+" terminals, causing it to record 0 Volts (C).
- Propagation will continue and eventually pass the voltmeter's "+" terminal, causing it to record a negative (downward) voltage reading (D).
- At some point, the impulse propagation will be complete, with the entire nerve fiber returned to its resting state and the voltmeter recording 0 volts. The Voltage vs. Time recording will then be complete (E).
Important practical note:
Since the voltmeter measures the differential voltage between its terminals, if the terminals come too close together, the voltage recording will be reduced in amplitude and a complete loss of recording ability may result. Keep this in mind when positioning terminal.
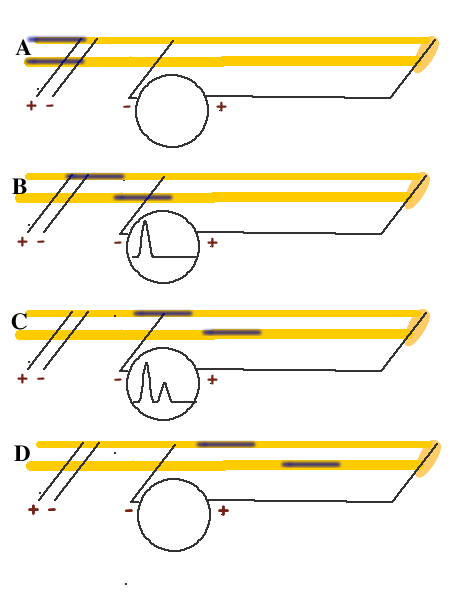
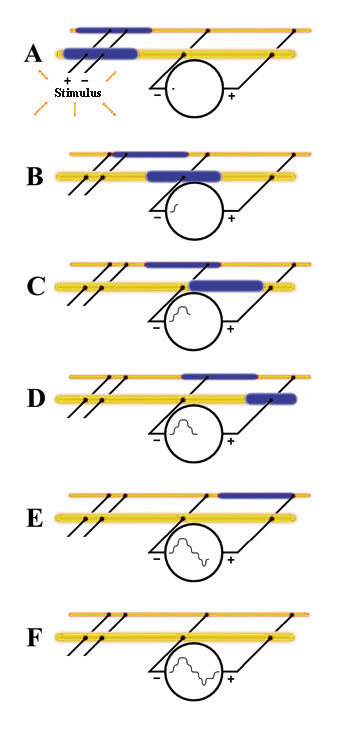 An actual CAP recording usesing a crab brachial nerve, which is made up of hundreds of individual nerve fibers, each with different diameters, results in different impulse propagation rates for each class of fibers. The resultant voltage recording is shown . This diagram shows six "snap-shots" in time, beginning with A and ending with F, to illustrate a theoretical connection to two individual nerve fibers.
An actual CAP recording usesing a crab brachial nerve, which is made up of hundreds of individual nerve fibers, each with different diameters, results in different impulse propagation rates for each class of fibers. The resultant voltage recording is shown . This diagram shows six "snap-shots" in time, beginning with A and ending with F, to illustrate a theoretical connection to two individual nerve fibers.
- Both nerve fibers receive the stimulus at the same time (A). However, since the two nerve fibers have different diameters, their nerve impulses travel at different rates. In general, the larger the nerve fiber diameter, the faster its impulse will travel. This difference is exagerated in unmyelinated fibers. (A)
- As each fiber's nerve impulse passes by the voltmeter's "+" and "-" terminals, the voltages add or subtract from each other to create the voltage plots. The fast fiber depolerization arrives at (B)
- The depolerization from both fibers add when both are present under the electrode at the same time (C)
- This "addition" results in C lowering the response at (D)
- At (E) only the impulse in the slow fiber is still present under the electrode.
- All diferences between the electrodes disapear as the action potentials move past the electrodes.
This biphasic recording begins to get very complex when several classes of slow fibers are just ariving at the first electrode while the faster fibers are passing over the second electrode. This problem can be overcome by recording "momophasic" action potentials. This is accomplished by by conecting the + electrode to the crushed end of the nerve. The action potential passes over the negative electrode but can not pass beyond the crushed portion at the positive electrode.
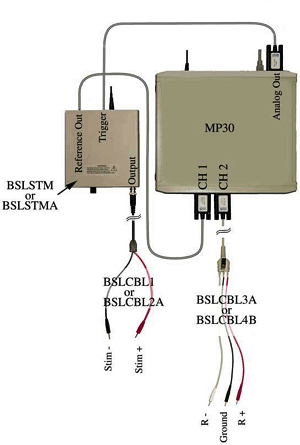 MP35 set up and connections for recording CAP:s
MP35 set up and connections for recording CAP:s
It is assumed that the MP3X and BSLSTMA are connected to their power sources (AC100) but are turned OFF. It is also assumed that the MP3X is connected to the host computer and that the BSL PRO software has been installed and is operational. Please refer to the Biopac Student Lab PRO manual for details.
Hardware setup
Connections to the BIOPAC Stimulator (BSLSTMx)
Connections to the Nerve Chamber
- Refer to the following connection diagram.
- "R" denotes the Recording cable (BSLCBL3A or BSLCBL4B).
- The "Ground" cable is the black lead of the Recording cable.
- The Ground leads of the Recording cable and Stimulator cable (Black, Stim-) should be connected to the same point on the nerve chamber.
- Special Notes:
- Lead placement may vary due to nerve length or the type of nerve chamber used. The leads need only
to follow the general order shown in these diagrams. That is, the positive response lead (Red - R+)
must be positioned further from the stimulator leads than the negative response lead (White - R-), but
both may be positioned on the same side of the nerve chamber.
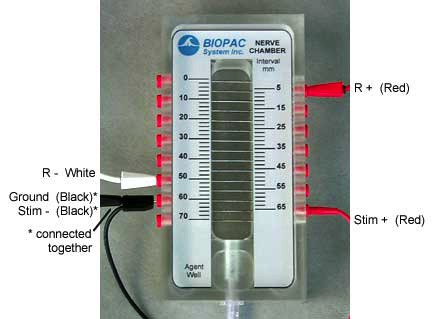 It is very important to have a solid electrical connection between the BSL leads and the nerve
It is very important to have a solid electrical connection between the BSL leads and the nerve
chamber. If there is corrosion on any lead or socket, it must be cleaned off. It is crucial to have a tight
fit between the lead and the socket.- Position the nerve chamber for the best possible access to it without bumping the nerve chamber or
pulling on cables or leads.
- It is important to have a good connection between the nerve and the pins in the chamber. One way to
assure good connections is to lightly abrade the top of the nerve chamber pins with an abrasive pad,
such as the BIOPAC ELPAD, and then clean them with alcohol.
Software Setup
- Launch the BSL PRO software. The program will automatically bring up a new "Untitled1" window.
- Move the template file (CAPtemp) from the network to your desktop and open it. Do not open the file on stushares
- The template will open to a graph window and the Stimulator window.
- Important! The Stimulator window must remain open during acquisition.
- Make sure BSL PRO is communicating with the MP3X unit.
- If working properly, there should be a green light next to the "Start" button
Software Setup
- Important! The Stimulator window in the software must remain open during acquisition!
- For a quick review on use of the BIOPAC Stimulator, please see the BSL Hardware Guide.
- Use the key to set the "Range" to 0 to 10 Volts (to the right).
- Flip the "Reference" switch to the "Actual" position (up).
- Turn the "Level" knob full counter-clockwise to set the Stimulator to 0 Volts.
- If the computer is not already ON, turn it on now, followed by the MP3X unit and then the BIOPAC Stimulator.\
- Check the LED display to confirm the Stimulator is set to 0 Volts.
- Make sure the stimulator is working by clicking on the "Start" button to begin an acquisition.
- Each time the recording is started, the output light on the front of the stimulator should blink.
- Note: When an acquisition is repeated you will get the warning "Overwrite existing data?"
Simply click on "OK." To turn this warning OFF, de-select the "Warn on Overwrite"
option from the MP3X menu.
 Stimulator Setup
Stimulator Setup
Important! The Stimulator window in the software must remain open during acquisition!
- For a quick review on use of the BIOPAC Stimulator, please see the BSL Hardware Guide.
- Use the key to set the "Range" to 0 to 10 Volts (to the right).
- Flip the "Reference" switch to the "Actual" position (up).
- Turn the "Level" knob full counter-clockwise to set the Stimulator to 0 Volts.
- If the computer is not already ON, turn it on now, followed by the MP3X unit and then the BIOPAC Stimulator.
- Check the LED display to confirm the Stimulator is set to 0 Volts.
- Make sure the stimulator is working by clicking on the "Start" button to begin an acquisition.
- Each time the recording is started, the output light on the front of the stimulator should blink.
Note: When an acquisition is repeated you will get the warning "Overwrite existing data?"
Simply click on "OK." To turn this
Calibration
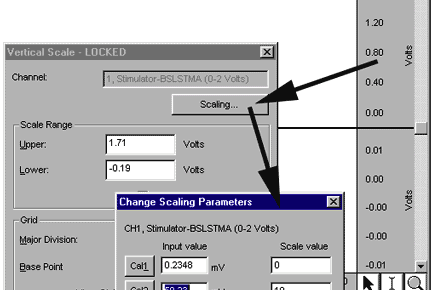 The MP3X needs to be calibrated with the "Reference Out" signal of the stimulator to make sure the baseline reading is 0
The MP3X needs to be calibrated with the "Reference Out" signal of the stimulator to make sure the baseline reading is 0
Volts.
- With the stimulator OFF (for BSLSTMx, pulse light on front of stimulator not illuminated or blinking), click the
mouse in the Vertical Scale region of Channel 1 to generate the Vertical Scale window. Click the "Scaling…"
button to generate the "Change Scaling Parameters" window. The initial settings should be as follows:
- Input Value 0 mV maps to Scale Value of 0 Volts
- Input Value 1 mV maps to Scale Value of 1 Volts
- Click on the Cal1 button. The Cal1 Input Value should now reflect the actual reading on Channel 1.
- Calculate the Cal2 Input Value (Cal2 Input Value = Cal1 Input Value + 1), and then manually enter it.
For example, if Cal1 Input Value =.23 mV, then Cal2 Input Value = .23 mV + 50 mV = 50.23 mV, so you
would type in 50.23. This example is shown below:
String as an Experimental Control
A thread can be used as an experimental control.You will see the stimulus artifact but no action potential response. The
artifact is created because the string has been made conductive by saturating it in Ringer's solution. Current can flow
across this conductive solution, so a response voltage can be measured. This is identical to what happens when stimulus
voltage is applied to the outside of the nerve. In the nerve this also initiates an electro-chemical response. The artifact
(electrical response) is detected ahead of the action potential (electro-chemical response). However, the action potential
response should be be of greater amplitude and of course it will be delayed due to the slower conduction velocity.
- Use a cotton thread that has been saturated with Ringer's solution.
- Cut the thread long enough to run the full length of the nerve chamber pin array but
not so long that it hangs down into the solution.
- Place the saturated thread along the length of the nerve chamber pin array.
- Add a drop of Ringer's solution at each pin-thread intersection (especially at the transducer points).
- Adjust the stimulator to a level of .5 Volts and "Start" an acquisition.
- Increase stimulator voltage in .5 Volt increments until desired data is displayed.
- Note that each time an acquisition is started, it will overwrite the existing data. To save desired data, use the
"Save As" option in the file menu.
- Remove string and return Stimulator "Level" to 0 Volts (full counter-clockwise).
 Nerve
preparation
Nerve
preparation
If all of the preceding procedures make sense , you are finally in
a position to begin your experiments. Your success with this experiment
depends on your ability to remove an undamaged nerve from a living crab.
Work quickly and carefully.
Caution The nerve is extremely delicate. Avoid excess stretching
during the removal process . Do not touch it with your fingers or anything
else; use the attached dactyl as a handle. Avoid excessive warming
- With a sharp scissors cut through the ischium of the swimming leg
of a large crab an collect blood in a weigh boat.
- Remove a walking leg from the crab by cutting through the coxa as
close to the body as possible.
- With a sturdy scissors cut the apodemes (muscle attachments) at
each of the joints in the leg.
- Firmly grasp the propodus and carpus and gently pull off the merus.
The nerve should remain attached to the next leg segment.
- Continue to pull off the remaining segments until only the nerve
remains attached to the dactyl (last segment).
- Place the nerve in very shallow pool of the blood you collected
above.
Mounting the nerve preparation
- Fill the bottom of the nerve chamber with ringers,but not so deep
that the fluid contacts the electrodes.
- Lay the nerve across the electrodes with the proximal end across
the stimulating electrodes, and the rest extending across as many
recording electrodes as possible, but without being unduly stretched.
- Depending on the length of the nerve, you may need to reposition the "R+" lead
such that it is connected to the pin that is closest to the end of the nerve, yet clearly in contact with it.
- The ends of the nerve should not hang into the chamber bed because they could end up contacting the excess Ringer's solution.
- Apply Ringer's across the entire nerve.
CAP Recording
Important: Irrigate the nerve with room temperature Ringer's solution between experimental applications to return to
baseline conditions, and begin each experimental recording with a baseline recording.
- Test the Setup
- Set the Stimulator to 0 volts.
- Click the "Start" button to record, then view, the first data segment.
- Stimulate at .1 Volt.
- Continue to increase in .1 Volt increments (up to 1 Volt max) until a CAP response is observed.
- Switch to Append Mode
- Append mode will append each successive recording segment onto the previous so that all recording segments will be
contained in one file.
- Choose "Setup Acquisition" from the MP3X menu.
- Choose "Append" from the second pull down menu
Monophasic action potentials
All of the traces that you have produced thus far are biphasic action
potentials. As the wave of depolarization reaches the first electrode
that electrode becomes more negative than the more distant electrode.
As the depolarization reaches the second electrode it is the one that
becomes more negative than the first. If a second class of fibers conducts
more slowly than the first its upward deflection can fall on top of
the downward deflection of the faster fibers. This can obscure
multiple peaks. A Monophasic CAP can alow visualization of several
classes of nerve fibers conducting at dirrering velocities. Note
that this is very different from a monophasic recording produced from
one electrode within the nerve and one on the outside. Monophasic compound
action potentials can be produced with the following techniques
- Completely deactivate the portion of the nerve lying over the most
distal recording electrode by crushing the nerve with a blunt probe.
- Place a drop of isotonic KCl (0.16M) on the crushed portion of
the nerve. Equalizing the potassium concentration inside and outside
of the axonal membrane in this fashion will inhibit the ability of
the cell membrane to depolarize and conduct an action potential past
this point.
- Connect the second recording electrode lead wire (red) to the most
distal recording electrode (under the deactivated portion of the nerve).
- Connect the first recording electrode lead wire to the recording
electrode 1cm from the stimulating electrode.
- Repeat the maximal voltage experiment and note that new peaks appear
as the voltage increases.
- Move the first recording electrode lead wire to the next most distal
electrode and again stimulate at increasing voltages.
- Move it to the most distal electrode (just before the crushed end)
- Acquire wave forms that most clearly demonstrates these additional
peaks (by varying the voltage and electrode position).
- What is the source of these additional peaks?
- Why do they spread out as the distance from the stimulus increases?
Conduction Velocity
You should have noticed that the nerve chamber has several additional
electrodes located down from the stimulating electrodes at one centimeter
intervals. This arrangement will allow you to make incremental changes
in the distance that an action potential must travel in order to be
recorded and allow you to determine the speed of conduction.
- Move the recording leads an additional centimeter further from the
stimulating electrodes. The action potential will now travel 2cm before
reaching the detection electrodes.
- Stimulate with single pulses at the maximal voltage determined above.
- You may note that the action potential is not as strong at this
distal point as it is proximally.
- How can you rationalize this in light of the all or nothing
law of action potential propagation?
- You may also note that the action potential has become wider.
- How can you explain this phenomenon.
- Record the results in a sequential file.
Amplitude, latent period, subthreshold, threshold, submaximal
maximal, and supramaximal stimulation
Using your nerve preperation investigate the effect of subthreshold, threshold, submaximal
maximal, and supramaximal stimulation on the amplitude and latency of the response. Think about latency and how you can tease out this value from your data.
Determination of threshold voltages
- Two of the interesting questions that we can ask about a nerve are
what is the minimum stimulation required to produce a response and
at what point does the nerve fail to produce a greater response to
increased stimulation.
- With the stimulator delivering 0.1ms impulses and the output control
set all the way off begin a series of acquisitions at increasing
voltages.
- You should increase the stimulus by about 10% for each experimental
run. At some voltage, usually less than 10, a new waveform should
appear to the right of the stimulus artifact. This is the action potential.
It should look somewhat like the idealized recording shown in Figure
5. You may have to go as high as 80% or 90%.
- The stimulus voltage at which a discernible action potential can
just be seen is the threshold voltage so fine tune the output untill
you are at the minimum setting that prodces a response.
- Make certain that you record the voltage and other important information
in the graph notebook before saving the file. The only way to
do this is to re open the acq file for the threshold run, enter the
data in the graph specific journal and resave the file. Changing
its name to Mac01Thresh will help find it again.
- Note any similarity to the response of the string?
Determination of maximum voltage
- Starting from the threshold voltage you just determined,continue
to increase the stimulus voltage, noting that the action potential
gets larger as you do so
- The increase in the action potential voltage may occur somewhat
irregularly. Why?. Why does it get larger if Action potentials
are all or nonme?. At the point where an increase in stimulus
voltage fails to cause a further increase in the action potential
voltage, a maximal stimulus has been delivered.
- Be certain that this value is also included in the notebook.
Again save as Mac01Max wopuld be a good idea.
Note: It is extraordinarily easy to produce
a fried preparation here.
Effect of heat and cold:
- Irrigate the nerve with warmed Ringer's solution (heat a beaker of Ringer's to not more than 40 deg
Centigrade).
- Start at 0 Volts and stimulate in .1 Volt increments until threshold is reached.
- Irrigate the nerve with cold Ringer's solution (use Ringer's from the freezer).
- Start at 0 Volts and stimulate in .1 Volt increments until threshold is reached.
Effect of nerve blocker: Topical lidocaine, procaine hydrochloride, or ether This could distroy the preparation do it last
- Establish the threshold for a response
- Irrigate the nerve with 10^-9M lidocane/ringers solution
- Wait aminute and reestablish the threshold for the same response
- Continue increasing the concentration by one order of magnitude utill the nerve no longer responds.
Report
Your report should incude your data reported as duration and magnitude
of each response to stimulation for each of the experiments that you
performed. Be certain to include the response of a non-living conductor,
the threshold and maximal voltages, the strength duration relationship,
the conduction velocity and the various classes of fibers that you can
demon-strate in the monophasic action potential. Include the data in
a well organized table where applicable. Use your own experience and
judgement as to the appropriatness of a graph. You should discuss how
your data compres to what would be expected from a vertebrate (your
text is a reasonable reference for this).
Walter I. Hatch
wihatch@smcm.edu
October 14, 2015
 Laboratory
6
Laboratory
6 Laboratory
6
Laboratory
6 The MP3X will be used to record the voltage difference between two electrodes with respect to time and simultaneously record the voltage across the stimulating electrodes. The diagram to the right shows five "snap-shots" in time of events occurring on a single nerve fiber beginning with A and ending with E. The recording is considered biphasic because the the voltmeter will record both a positive and negative deflection.
The MP3X will be used to record the voltage difference between two electrodes with respect to time and simultaneously record the voltage across the stimulating electrodes. The diagram to the right shows five "snap-shots" in time of events occurring on a single nerve fiber beginning with A and ending with E. The recording is considered biphasic because the the voltmeter will record both a positive and negative deflection.
 An actual CAP recording usesing a crab brachial nerve, which is made up of hundreds of individual nerve fibers, each with different diameters, results in different impulse propagation rates for each class of fibers. The resultant voltage recording is shown . This diagram shows six "snap-shots" in time, beginning with A and ending with F, to illustrate a theoretical connection to two individual nerve fibers.
An actual CAP recording usesing a crab brachial nerve, which is made up of hundreds of individual nerve fibers, each with different diameters, results in different impulse propagation rates for each class of fibers. The resultant voltage recording is shown . This diagram shows six "snap-shots" in time, beginning with A and ending with F, to illustrate a theoretical connection to two individual nerve fibers. MP35 set up and connections for recording CAP:s
MP35 set up and connections for recording CAP:s It is very important to have a solid electrical connection between the BSL leads and the nerve
It is very important to have a solid electrical connection between the BSL leads and the nerve The MP3X needs to be calibrated with the "Reference Out" signal of the stimulator to make sure the baseline reading is 0
The MP3X needs to be calibrated with the "Reference Out" signal of the stimulator to make sure the baseline reading is 0 Nerve
preparation
Nerve
preparation