 Appendix
E
Appendix
E Appendix
E
Appendix
E
Corning 2655-00 Digital Flame Phometer
.
This instrument is a clinical flame photometer. It was designed to provide very rapid moderately accurate (1-2%) readings from clinical samples like blood, sweat and tears with the occasional lot of urine. It was designed for routine operation with little training. The clinical design parameters limit its ease of use in non clinical setting where values nm ay be outside the range of the instrument. With some attention to detail in sample preparation it's use can be broadened to encompass the animal physiology lab. This unit is a single chanel, low temperature flame fhotometer for the determination of Na K Li and Ca. It has been fitted with a lineizer module which electronically straightens the calibration curve and permits single poin calibrations.
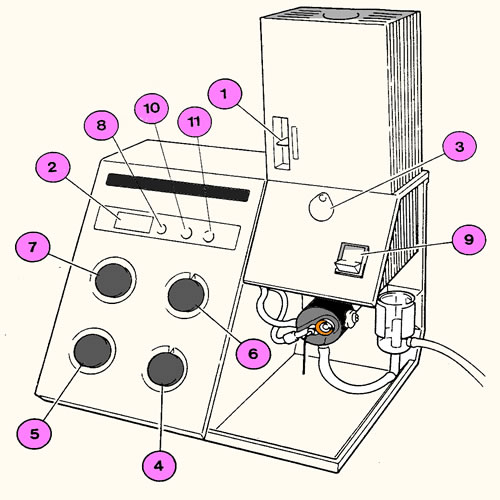 Power
On (10)
Power
On (10) Omit this section if the instrument is already operating
Check to be certain that the gas and air supply lines are securely attached to the instrument.
Turn on the gas supply at the tank and verify that the tank is providing 30 psi. Turn the gas supply back off for now.
Turn on the air supply (lab air tap on the bench) and adjust r at the rear of the instrument until the air pressure gage indicates 12 psi.
Verify that the waste water waste water "U" tube is filled with water. Fill it if the level is low. See figure 3 for the location of the U tube.
Turn the fuel control fully clockwise to the closed position, but do not force it. This control is a precision assembly that will be irrevocably damaged by rough handling.
Open the fuel control 9 turns (counterclockwise)
Omit this section if the instrument is already operating
Depress the power switch on the front panel. The LED will light and an ignition sequence will commence.
Recheck and readjust the air pressure regulator on the rear panel until the gage reads 12 psi.
Turn on the fuel at the source
Important:: If the flame on LED has not illuminated before the end of the ignition cycle, switch off and wait 60 seconds. Then with out adjusting the fuel control switch on again to initiate another cycle.
If ignition fails again, open the fuel control 1 additional turn and retry. You may repeat this procedure until ignition occurs but under no circumstances should you open the fuel control more than 4 additional turns (to 13 turns) or explosive concentrations of gas may accumulate.
Switch to the ion of interest by sliding the selection lever indicate Na, K or Ca. Make sure that the drainage water-trap is full by adding distilled water from a squeeze bottle until it can be seen running out of the overflow tube.
Insert the nebulizer tube into a beaker containing at least 100 ml of dilutant and continue to aspirate the dilutant for 15 minutes while the instrument warms up. Accuracy is achieved only after a 15 minute warm up.
Immediately before use, working standards are prepared by diluting the stock standards above with distilled water to produce a 10mM working standard. (299.9 ppm for Na and 399.0 for K) One hundred ml will serve the entire class, so check each others calculations and make up a single batch for each ion. Remember that you need to set the value 10.0 (using the calibration dials) while the sample is asperating
It is critical to remember that both your samples and this working standard must be diluted 1:200 (25 ul stock in 5000 ul diluent immediately before aspirating it into the flame. Full strength working standards or blood samples can not be read and they will will contaminate the aspirator. The blank working standard is pure dilutant with neither sodium or potassium added serves as the 0 mM blank for adjusting the 0 value of the instrument. Although working stock solutions will keep for about six months at 4∫ C, it is not recommended. Working standards should be held for no more than a week, nor made up in quantities of more than 500 ml.
Select the appropriate filter for your ion and, while aspirating dilutant, adjust the blank control so that the display reads zero.
Aspirate your presentation standard (working standard diluted 1:200 with diluent). Allow 20 seconds for the reading to stabilize , and then adjust the course and fine controls to the value of the standard (10 mM for both Na and K). Note: the decimal point. This simply lights up the dot it does not change anything else but it will be easier to read the instrument if you light it up. Your 10.0 mM standard can now be adjusted to 10.0 instead of 100
Remove the standard and aspirate a blank solution of dilutant for 20 seconds. Readjust the blank control.
You can aspirate any other calibration standards that you may have prepared for the same ion and produce a multipoint calibration curve. Start with the lowest and work toward the highest to avoid carry over. Aspirate for 20 seconds, read the instrument then aspirate diluent for 10 seconds and aspirate another sample.
This instrument is equipped with an computer linearizer which lessens the need for preparing a standard curve. In order to obtain maximum linearity from this instrument, however, for maximum accuracy your standards should be near the middle of the effective range for each ion. Remember that you must change the filter to match each ion.
Carry out your tests, rechecking zero and standards as necessary, or at intervals of not more than 10 to 20 minutes in any case. Remember that you must dilute your samples exactly as you did your standards in order to produce accurate readings. If your readings are exceptionally low (<2.0 mM), your accuracy will suffer and you should consider consider rediluation to a more appropriate concentration. If you dilute 1:100 instead of 1:200 you will double the effective concentration seen by the machine. Don't for get to correct your values for the dilution factor. Remember that the effective concentration range of instrument is from 0 to 200 mmol/L. At no time can a reading greater than 200 be considered accurate. It must be further diluted and the result corrected for the dilution factor.
In addition, This instrument is very good at rejecting interference from other ions. but high levels of any ion will affect the determination of the others. The following specifications will help you deter mine if you are apt to experience interference.
Na - Errors in Na readings due to Ca, K or Li present in biological fluids will not exceed 1 mmol/L Na where the levels of these elements do not exceed 3.5 mmol/L Ca, 100 mmol/L K, and 2.0 mmol/L Lie
K - Errors in K readings due to Ca, Na or Lie present in biological fluids will not exceed 0.1 mmol/L K where the levels of these elements do not exceed 3.5 mmol/L Ca, 180 mmol/L Na, and 2.0 mmol/L Lie
The over all accuracy of this instrument is better than 2%