 ory
04
ory
04 ory
04
ory
04The primary objectives for this laboratory are
Understanding the flow of mater and energy through ecosystems is one of the primary foci of ecologists. While the flow of energy is a one way process, mater recycles through both the living and physical components of the biosphere. Understanding these cycles requires accurate determination of the levels of atoms and molecules that are critical to living systems. These critical materials are of course nutrients. Inasmuch as autotrophic organisms form the base of the food webs, it is often the level of nutrients available to these organisms that set the limits on growth in any environment. Understanding the sources and sinks of plant nutrients as well as the details of their biogeochemical cycles are, therefore, of considerable interest to ecologists. Understanding natural capital and the ecological services of costal wetlands should be of considerable interest to everyone. Autotrophic organisms require numerous micro and trace nutrients. Autotrophs living in aquatic environments, especially marine and estuarine environments are continuously exposed to sufficient quantities of trace and micro nutrients to meet their needs. It is more parsimonious to assume that nutrients required in bulk are more likely to limit plant growth in costal wetlands. Magnesium, calcium, potassium and sulfur are required in relatively small amounts and are abundant in sea water. Iron is a bit more variable but not often limiting. This leaves us with phosphorus and nitrogen; no surprise to those of you who have read a fertilizer bag and discovered the code. The levels of N, P and K are clearly marked on the label (10 5 10 for example). Note: although K can become limiting in terrestrial environments it is unlikely to limit marine or estuarine waters.
Nitrogen occurs in natural waters and in wastewaters as ammonium (NH4), nitrite (NO2), nitrate (NO3) and various organic compounds. It occurs in solution, in particles of detritus, or in the bodies of aquatic organisms. While determining the levels of each of the nitrogen species may help identify its source, most aquatic plants use N in all of the inorganic form forms mentioned above. So, you will need to measure ammonium (NH4) and nitrate (NO3) and calculate the total available nitrogen in order to reflect limiting factors for autotrophic growth, biological activity, or changes in the natural chemistry of waters. (Note: the MO3 assay will report total NO2 and NO3 =NOx) In addition total N may serve as an important indicator of pollution and eutrophication.
Phosphorous occurs in natural waters and in wastewaters almost solely as phosphates. These are classified as orthophosphates, condensed phosphates (pyro-, meta-, and other polyphosphates), and organically bound phosphates. They occur in solution, in particles or detritus, or in the bodies of aquatic organisms. Like Nitrogen the various forms of phosphate arise from a variety of sources. Orthophosphates applied to agricultural or residential cultivated land as fertilizers are carried into surface waters with storm runoff and to a lesser extent with melting snow. Human activity also contributes to phosphate additions. Small amounts of certain condensed phosphates are added to some water supplies during treatment. Larger quantities of the same compounds may be added when the water is used for laundering or other cleaning, because these materials are major constituents of many commercial cleaning preparations. Phosphates are used extensively in the treatment of boiler waters. Organic phosphates are formed primarily by biological processes. They are contributed to sewage by body wastes and food residues, and also may be formed from orthophosphates in biological treatment processes or by receiving water biota. Orthophosphates also occur in bottom sediments and in biological sludge, both as precipitated inorganic forms and incorporated into organic compounds. Phosphorus analyses embody two general procedural steps: (a) conversion of the phosphorus form of interest to dissolved orthophosphate, and (b) colorimetric determination of dissolved orthophosphate. We will determine only the reactive orthophosphates in this class; not because the poly and organophosphates are inconsequential but because they are not directly used by most plants and the conversion of these compounds to measurable orthophosphates is very dangerous and very time consuming.
Historically the measurement of nutrient concentrations required considerable time, training and quantitative skills. Fortunately you will use a system of prepackaged chemistry coupled with a dedicated spectrophotometer/computer developed by Hach. This system is based on the chemistry of standard analytical methods, but the prepackaging of the reagents and pre-programed calculations allows a very much faster analysis and it is much more accessible to untrained analysts. In addition field operable spectrophotometers allow onsite field determinations and eliminate the storage and transpiration problems associated with some nutrient analyses. You will find the Hach DR/2400 Users Manual in the laboratory appendices. You should look through the manual and learn how to turn it on and off and what the buttons do. The assays you will perform are presented in the Low level Ammonia assay, Mid level NO3 assay, and Low level reactive phosphate. Each lab team will collect water and determine the nutrient levels in triplicate samples from your study sites.
Site assignment will be made in class and the data shared. There are 4 sites so you should divide up into 4 teams, one for each of the sites to be sampled.
The Hach spectrophotometers and protocols were designed to run one assay (in its entirety) at a time. Every thing including the timers is built into the program. While we could proceed with strict adherance to the manual this will result in trafic jmbs at the 2 specrrophotometers. This can be eliminated by conducting all of your assays in plastic disposable centrifuge tubes and then transfering the contents to the Hach cuvette for spectrophotometric analysis. The colors are stable bor a few hours so there is no rush to read the results. In order to do this you need to measure out your sample and blank into the centrifuge tubes and the proceed as instructed using a separate timer rather than the one in the specs.
You should run the PO4 assay first, the NOx assay next ann the NH3 assay last. This is in the order of complexity of the assay and provides some experience for running the more difficult annonia assay.
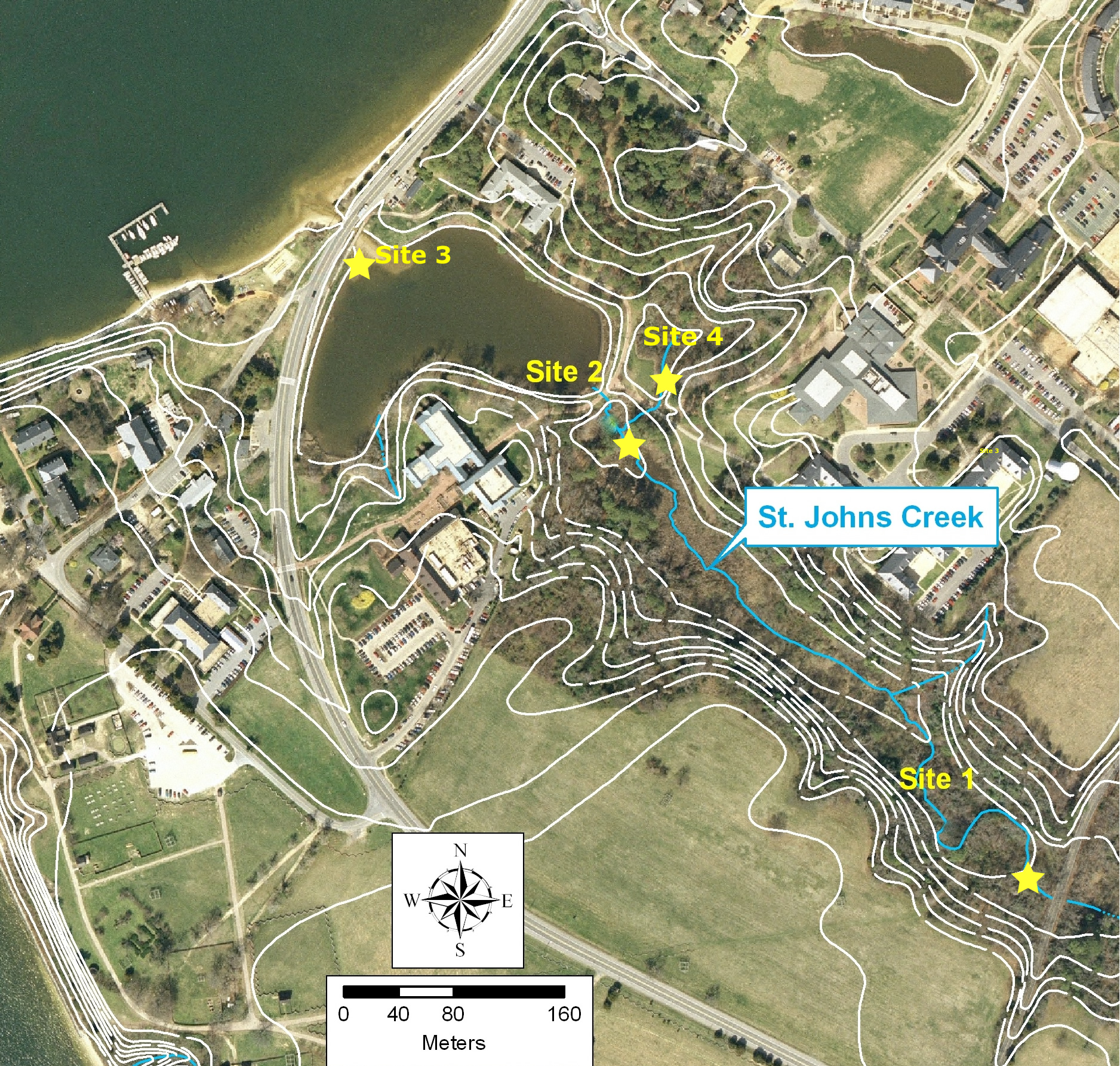
You should submit a table with the average nutrient concentratins (NOx, PO4, and NH4) at each site. You should discuss these values with in the context of your hypothesis on the source and fate of each of these nutrients. If the values are increasing or decreasing speculate on why and how. What do you think are the sources and sinks at each station..
Walter I. Hatch
wihatch@smcm.edu
October 22, 2013